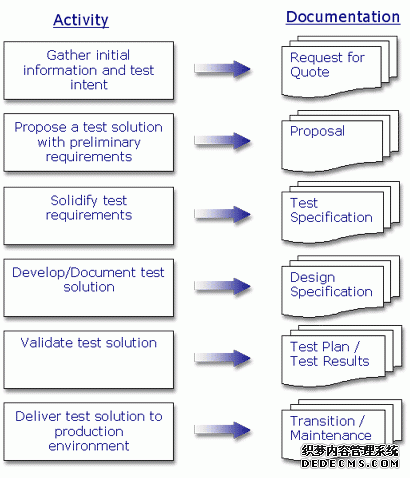
Test development activities follow documented procedures. Processes have been refined from experiences to meet relevant standards such as ISO9001-2000, TL9000, EN46001 and FDA medical device standards
Test Development Process Overview
Development activity is focused on test requirement analysis and documentation in the form of a test specification. Design Verification activities included design reviews at key points during the design. Reviews include organized review by peers of the hardware, fixture, and software associated with this test development. The reviews and other key activities are called out in a project plan. As an ISO9001 company, projects are not complete without a validation process. A Test Plan is written, approved, and implemented to fulfill the validation phase.
Documentation Diagram